by
Carol Ko, Staff Writer | March 11, 2013
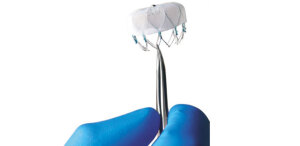
Boston Scientific's
Watchman device.
Thousands of attendees gathered at the American College of Cardiology Scientific Sessions on Saturday morning to hear the widely anticipated results of a study on a new heart device which, if approved for sale in the U.S., could change the way doctors treat stroke prevention.
There was just one problem: the announcement was pulled at the last minute.
Boston Scientific sent an investor relations email at 6:30 a.m. Eastern Time reporting the key results of its Prevail study, breaking the ACC's embargo. "It was an inadvertent mistake for which my team is apologetic and regretful," Denise Kaigler, senior vice president of corporate communications at Boston Scientific, told DOTmed News.



Ad Statistics
Times Displayed: 178984
Times Visited: 3218 For those who need to move fast and expand clinical capabilities -- and would love new equipment -- the uCT 550 Advance offers a new fully configured 80-slice CT in up to 2 weeks with routine maintenance and parts and Software Upgrades for Life™ included.
When the blunder forced the ACC to cancel the announcement of the study results, a murmur rippled through the audience.
"I can't believe it," session attendee Laura Gonzalez whispered, reading breaking news of the canceled announcement on her smartphone.
Days before the conference, Boston Scientific had issued a release saying it wouldn't be presenting the full results of the trial. Then, in a highly unusual reversal, the device firm announced that it would be presenting the full results after all.
While the study suggested that the Watchman device may be safer than previous studies had found, it failed to prove that the device was any more effective than anticoagulant medication on lowering the occurrence of strokes, heart-related deaths or embolism at the 18 month point.
The snafu comes as Boston Scientific continues to experience a revenue slump in its core product line of defibrillators and stents. The Watchman device is one of several new products that the company had hoped to introduce in a bid to drive growth and reverse its fortunes.
Prevail trial background
The U.S. Food and Drug Administration initially refused to approve the Watchman device in 2010, citing concern over procedural complications associated with the device during its initial trial.
The randomized trial that followed in response, known as the Prevail trial, tracked 407 patients who suffer from stroke-inducing blood clots who were either fitted with the device or treated with anticoagulant medication - currently the most common treatment for patients.
But anticoagulant medication has numerous limitations of its own that cause patients to opt against it. Researchers are looking into alternative devices like Watchman in hopes that it might improve stroke prevention.

