by
Barbara Kram, Editor | December 15, 2009
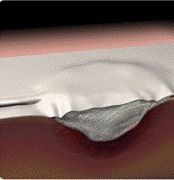
Negative pressure dressing
FDA has notified health care providers and patients of rare but serious complications including deaths from the use of negative pressure wound therapy (NPWT). These devices are used to speed healing of acute or chronic wounds or burns by closing wounds with a vacuum in sealed wound sites. A vacuum pump creates sub-atmospheric pressure to remove fluids and bacteria, promote blood flow, and draw the wound edges together.
Over the past two years, FDA has received six death and 77 injury reports associated with NPWT. Most deaths occurred at home or in long-term care facilities. Bleeding was the most serious complication. Extensive bleeding has occurred in patients with blood vessel grafts in the leg, with breastbone or groin wounds, and those receiving medication for blood clots during removal of dressings attached to the tissues.
The reports also included cases of infections from original open-infected wounds worsening due to pieces of dressing that remained in the wound, and of injury from foam dressing pieces and foam sticking to tissues or clinging to wounds. Most of these patients required surgery, additional hospitalization, and antibiotics.
FDA is addressing these problems and will continue monitoring adverse events associated with NPWT devices.
Get all the FDA details and recommendations:
http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm193277.htm
A market forecast is available from Research & Markets:
http://www.researchandmarkets.com/research/e5bac9/negative_pressure
