Precision cancer care with proton and radiation oncology
October 29, 2018
by Lisa Chamoff, Contributing Reporter
In cancer treatment, time is of the essence. For the best outcomes, precision is also a key component. Whether it be proton therapy or conventional radiation oncology, those goals are at the heart of the new treatment systems and oncology software entering the market.
The rise of proton therapy
Proton therapy has had a slow adoption due to the high price tag, but as evidence accrues for its benefits and insurers begin to take notice, access is coming within reach for more of the patients who stand to benefit most from it.
Hitachi
The company has been moving ahead with installations of its Hitachi Proton Beam system since receiving FDA clearance in 2008.
The system is equipped with a compact synchrotron accelerator, which the company says allows for beam delivery at selected energy levels, with significantly less neutron generation, and is used by facilities including the MD Anderson Cancer Center and Mayo Clinic sites in Minnesota and Arizona.
The company is currently working on an installation at Sibley Memorial Hospital, a member of Johns Hopkins Medicine, in Washington, D.C., and hopes to start treating sometime in late 2019, said Sash Matsumoto, general manager at Hitachi America.
The company also announced late last year that it will install a system at Clinica Universidad de Navarra (CUN) in Madrid, Spain.
Matsumoto noted that the lead time for constructing proton therapy facilities is getting shorter and the overall cost is coming down, though facilities are mainly focusing on one- or two-room centers instead of multiple rooms because of the investment.
“Not every hospital can afford a multi-room proton system,” Matsumoto said. “I think every vendor is doing everything we can to lower the cost and, hopefully, it will allow more hospitals to have the technology. With more clinical trials providing evidence, it’s become more and more clear that for certain tumors proton is the best treatment and it’s something that every patient deserves to have access to.”
IBA
Since the company’s ProteusPLUS treated its first patient in 2001 and its smaller ProteusONE was first used in 2014, IBA has been rapidly deploying its technology. In the last six months, IBA completed four installations of the ProteusONE – which has pencil beam scanning and cone beam CT – around the world.
The company’s goal is to make the system such that it could be used for a wide variety of indications, with the ability to treat complex tumors, including moving ones, using respiratory gating systems and better imaging, said Nicolas Denef, IBA’s product management director.
Denef said the ProteusONE, which has a smaller footprint and offers an affordable solution for a one-room proton therapy system, has become the preferred technology as fewer facilities have built multi-room facilities.
Several years ago, developers thought that having four to five rooms would be more financially beneficial, but patient recruiting was more difficult for more remote Proton Therapy Centers.
“There was an idea that if you build it they would come,” Denef said. “Which happened for some, but not for the ones that were far away from city centers.”
The company’s IBA Dosimetry solution decreases the time needed for beam data commissioning and accelerator QA, Denef said.
In the future, IBA is looking to continue to improve imaging and introduce improved workflows, Denef said. The ultimate goal is to do adaptive treatment, using the cone beam CT to replan quickly just before treatment starts.
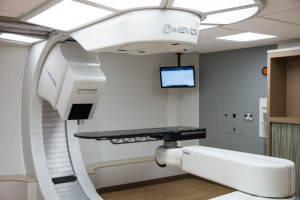
Mevion Medical Systems
Earlier this year, Mevion received FDA clearance for HYPERSCAN pencil beam scanning for its compact MEVION S250i Proton Therapy System. Shortly afterward, MedStar Georgetown University Hospital in Washington, D.C., became the first to treat a patient with the system, which combines pencil beam scanning with a single-room machine.
The system features what Mevion calls the Adaptive Aperture proton MLC, which the company says generates layer-by-layer specific beam collimation and blocking with beam sharpening performance identical to conventional proton therapy apertures.
“The combination of HYPERSCAN and Adaptive Aperture moves the science of proton therapy ahead to even sharper dose distributions,” said Skip Rosenthal, vice president of clinical systems for Mevion Medical Systems.
The company has two more systems in deployment, one in Europe and another system being installed in the U.S. in the fall.
An important aspect of the HYPERSCAN is the ability to have support from advanced treatment planning, and the company has a collaboration with RaySearch. Currently, centers will be using RayStation, the RaySearch treatment planning system to provide clinicians with Monte Carlo-based planning for HYPERSCAN. Rosenthal said that by the end of the year, RaySearch will release the ability to plan and treat using the full multilayer collimation capabilities of Adaptive Aperture.
Rosenthal said Mevion also is integrating cone beam CT and CT on rails with the system sometime this year.
“The ability to use that in a proton room has been slow to materialize,” Rosenthal said.
P-Cure
P-Cure has marketed its gantry-less solution, and the company is planning to soon publicize results from use of the P-Cure system on patients being treated for lung, brain, eye and other types of cancer at the Northwestern Medicine Chicago Proton Center.
“The objective is to show … that patients can be treated even better without a gantry, in the seated position,” said Michael Marash, P-Cure’s chief executive officer. “When a patient is seated, the magnitude of the inner organ motion is minimized, enabling it to direct the beam to the tumor with much greater precision. There are also fewer equipment and construction costs, as a gantry needs three floors.”
At the Chicago center, P-Cure enabled treating in their open registry study. Next year, the company will look to show the effects of the system on treating several other tumor sites including, head and neck, breast, and liver cancers.
The company is also starting to explore international markets and has received a positive response from facilities in India and China, Marash said. The company is building a new facility in the center of Israel to ship both west and east.
ProTom International Holding Corporation
ProTom has made moves into the Asia Pacific market and was recently awarded the contract for the Australian Bragg Centre for Proton Therapy, the first proton therapy center in Australia, to be housed in a massive new health complex expected to break ground in the second quarter of next year in Adelaide.
“The U.S. market is more mature,” said Stephen Spotts, chief executive officer of ProTom International. “As cancer providers are looking at adding proton therapy, more providers are looking at single room systems.”
ProTom's Radiance 330 Proton Therapy System – which received FDA clearance in March 2014 – uses one synchrotron accelerator for multiple rooms, which is different from the company’s competitors, except for Hitachi, Spotts said. The center in South Australia is using renewable energy, which is one of the reasons they chose ProTom, which uses a synchrotron, with varied acceleration levels, as opposed to a cyclotron, where the acceleration is constant.
“Our energy consumption is about 30 percent of a multiple-room cyclotron,” Spotts said.
Radiation oncology
Like proton therapy, radiation oncology companies have shown greater interest in simplifying their systems while also providing greater specificity. What follows is a look at some of the newest software and hardware solutions from companies in the conventional radiation oncology space.
Accuray
At this month’s ASTRO annual meeting, Accuray will be introducing the CyberKnife VOLO optimizer for the CyberKnife robotic radiation therapy system. The system simplifies the process of creating treatment plans using a new next-generation optimization algorithm, said Corey Lawson, vice president of product strategy at Accuray.
“It’s a state-of-the-art optimizer that resets the bar for user experience,” Lawson said.
Lawson said VOLO should reduce optimization time, or the time to do calculations, by 95 percent. So, a lung cancer case that may have taken close to an hour with the previous optimizer will now take a few minutes. It will also reduce delivery time by more than 20 percent.
Faster treatment planning means clinicians can try different ways of planning a case with much greater efficiency, Lawson said.
“With the ability to push a plan harder than ever before, will likely come the ability to get better plans than previously thought possible with the CyberKnife System,” Lawson said. “We expect our clinical partners to readily adopt this new technology because of the clinical workflow and plan quality benefits that will be quickly realized.”
Accuray also recently introduced CTrue IR imaging on its Radixact Treatment Delivery System. CTrue IR integrates a new iterative reconstruction algorithm which improves soft tissue contrast, while also reducing noise in scanned image sets.
"It does all of this with the same low-dose imaging and fast scan time we've always had,” Lawson said. “Furthermore, it is not affected by metal artifacts, nor in larger patients, the obscuring effects of electron starvation. We are truly improving user experience and clinical utility with this new release for our Radixact System.”
The company is also planning to show Synchrony motion compensation on the Radixact Treatment Delivery System, which is 510(k) pending at the time of this article.
Lawson stressed that the new feature is very different than conventional gating solutions. It will use an algorithm to adjust the beam to follow a moving target throughout its motion cycle, in real-time.
Brainlab
Brainlab released a newer version of its Elements Multiple Brain Mets SRS treatment planning software for focused stereotactic radiosurgery. The updated software provides new treatment strategies to treat brain metastases and adds new contouring and response assessment tools to increase utilization, said Bogdan Valcu, director of clinical research at Brainlab.
The previous version of Elements Multiple Brain Mets SRS was only planning for a single fraction radiosurgery, or the treating oncologist would have to specify one fractionation strategy, Valcu explained. With the new version of the software, you can combine multiple strategies, with one tumor receiving a single fraction and one receiving multiple fractions.
“We just want to provide more flexibility within this application, enabling everything from single fraction radiosurgery to fractionated SRS prescriptions customized to the individual patient disease and irrespective of tumor size,” Valcu said. “Using Elements Contrast Clearance Analysis, we can now detect earlier whether or not patients are failing therapy and require re-treatment. Timely re-treatment is critical for maximizing survival profiles for brain metastasis patients and so is the ability to distinguish between tumor and radiation effect.”
The spine is another key target for Brainlab, and in March 2017 the company released Elements Spine SRS. First patient treatments began in Argentina, Germany and Italy followed by the first patient treatment in the U.S. in August of 2018.
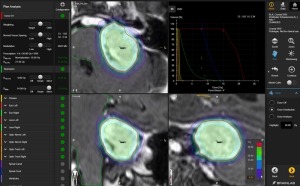
Elements Spine SRS works with CT and MR scans to calculate the clinical target volume using international spine radiosurgery consortium consensus guidelines, and takes less time using Monte Carlo simulations.
“The software offers automatic treatment planning, which helps raise overall plan quality with consistent adherence to desired treatment protocol. In a half hour clinicians can go from zero to treatment plan,” Valcu said. “Planning steps that would have taken clinicians two to three hours to perform in the past are now performed in under 10 minutes and physician involvement time is reduced to just a few minutes.”
Brainlab specializes in customized algorithms for the treatment site, working around the challenges of the spinal column.
In March 2017 Brainlab also released Elements Cranial SRS for treating primary tumors in the brain. The software corrects the cranial distortion from MR scans, Valcu said.
“Planning automation is paramount for this software, starting with tools to correct MRI distortions and improve co-registration, to planning CT and continuing with a comprehensive risk organ definition based on a tissue-class universal atlas,” Valcu said.
The company plans to continue developing indication-specific solutions and plans to focus on metastases in the lung and liver and other body sites.
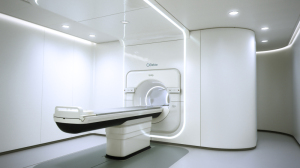
Elekta
Elekta has mainly been promoting its Elekta Unity MR-linac, which received a CE mark in June 2018 (the company submitted for FDA 510(k) clearance in beginning of August).
In August, the first treatment of a cancer patient with a recurrence in a pelvic lymph node with the Unity was completed in the Netherlands. The concentrated high-dose treatment took 10 days, significantly less than typical radiation therapy treatments that require up to 30 fractions, said Ioannis Panagiotelis, the chief Marketing and sales officer for Elekta.
On the software side, Elekta released a product called AQUA, which standardizes and automates clinical quality assurance tests.
The company also entered into two agreements. One is with IBM, to allow its Watson for Oncology to pair with Elekta’s MOSAIQ, oncology information system and personalize treatment with artificial intelligence (AI). Elekta also partnered with Palabra to allow clinicians to use voice commands for MOSAIQ to expedite clinical operations and reduce the time needed for data entry.
“Our customers have access to the latest and greatest when it comes to software solutions that have been developed by other parties,” Panagiotelis said.
RaySearch Laboratories
Late last year, RaySearch Laboratories released RayCare, its new oncology information system (OIS).
Johan Löf, president and chief executive officer of RaySearch Laboratories, said RayCare is a “completely new take on” the OIS, with an “advanced architecture that is extremely resilient and scalable.”
Users have full access to the system through Android or iOS devices and the company introduced a structured way of handling workflows. If a user is logged on in a certain role, they will only see the tasks relevant to that role. The company has been updating RayCare to add functionality, including billing.
In June, the company released a new version of its RayStation treatment planning system that includes full planning capabilities for the TomoTherapy and Radixact systems from Accuray, and support for Accuray’s TomoDirect and TomoHelical delivery modes.
Later this year, RaySearch Laboratories plans to release an auto-contouring product that applies deep learning, with a database of “well-contoured” patients that will allow the process to be completely automated.
Varian
Varian recently released version 2.0 of its Halcyon radiotherapy system, which debuted at last year’s ESTRO meeting in Vienna.
The new version adds additional imaging modalities – kV cone beam CT and iterative cone beam CT, which Tracey Fisher, director of marketing for the Americas oncology division at Varian, said brings a “diagnostic-like” quality to the images.
“What is unique about Halcyon is the speed,” Fisher said. “You can get kV volumetric images in as few as 17 seconds. It’s better soft-tissue imaging and target delineation. The more you can see, the better is the potential treatment.”
The Halcyon is field upgradable, and since the launch Varian has taken 98 orders for the system worldwide.
The new version of Varian’s Eclipse treatment planning system, Eclipse 15.5, enables many of the features of the Halcyon 2.0 and a feature that allows clinicians to more easily adapt a treatment plan from one machine to another. Additionally, Eclipse 15.5 features Multicriteria Optimization (MCO), which allows clinicians to explore what happens when different clinical criteria are varied, such as the degree to which particular organs are spared versus coverage of the targeted tumor.
In April, Varian announced a new version of its Velocity cancer imaging software. Called Velocity 4.0, it has several new capabilities, including RapidSphere image-guided Y90 dosimetry, which offers an image-guided dosimetry solution for Y90 Selective Internal Radiation Therapy (SIRT) and is a method for tracking absorbed dose, confirming that the treatment was delivered as planned.
The software also offers new features including Velocity M3i, which allows clinicians to use images from multiple modalities – CT, MR, PET, SPECT and ultrasound – for contouring, and Velocity ARIA Sync, which automatically synchs with Varian’s ARIA oncology information system and Eclipse treatment planning system.
A feature called Velocity Tumor & Dose Tracking, which can track radiation dose of multiple treatments and different types of therapies, allows clinicians to verify volumetric tumor changes and a patient’s cumulative dose history.
“This allows them to more easily account for prior treatments and their impact on current or future treatments,” Fisher said.
ViewRay
Last year, ViewRay’s big release was the MRIdian Linac, which combines a linear accelerator with an MR scanner and was cleared by the FDA in February 2017. The company later announced imaging improvements under development that double the signal-to-noise ratio, frame rate and resolution without raising the field strength, said Jim Dempsey, ViewRay's chief scientific officer.
The device uses what Dempsey called a “proprietary approach” to compressed sensing.
“We think there are some very impressive imaging improvements, under development,” Dempsey said. “Speed, resolution and SNR are potentially improved by a factor of three.”
Elekta, which released a similar MR-linac, called the Elekta Unity, uses an MR scanner with a higher field strength
The second generation is available via an upgrade, and Dempsey said some customers with service agreements have already purchased upgrades.
“Their argument has been you need high field strength,” Dempsey said. “Elekta and Philips made a decision to sacrifice dose quality for a better image. As a small team, we work on impossible problems, such as how to make a low-field MRI work like a high-field MRI.”
Dempsey notes that the clinical data shows that the MRIdian can treat cancer with lower toxicity.
“The biggest headlines for us are the clinical data,” Dempsey said.
Xstrahl
In December 2017, Xstrahl launched the new RADiant X-ray radiation therapy system with an FDA 510(k) clearance, which was followed by a CE mark in March 2018.
The RADiant system offers two types of treatment – electronic brachytherapy (eBX) and superficial radiotherapy (SRT), which is different from conventional radiotherapy systems currently on the market, according to Adrian Treverton, chief operating officer of Xstrahl Inc. This allows customers to increase their throughput and allows for adoption by the American dermatological market, which cannot use the high-powered radiotherapy systems currently on the market.
“The radiotherapy industry current has two main trends – a significant increase in skin cancers and skin conditions worldwide, and increased pressures on departments and clinics to treat patients in cost- and time-effective ways,” said Treverton. “The RADiant offers a cost-effective treatment platform treating not only skin cancers, but also non-malignant skin conditions, keloids and dermatological conditions, allowing for reduced pressure on oncology departments, and an increase of client base for dermatological clinics.”
RADiant is currently installed at four sites across the continental U.S., both in radiotherapy oncology departments and dermatology clinics. The RADiant will also soon be released in Europe, South America and China, before a full worldwide rollout.
The rise of proton therapy
Proton therapy has had a slow adoption due to the high price tag, but as evidence accrues for its benefits and insurers begin to take notice, access is coming within reach for more of the patients who stand to benefit most from it.
Hitachi
The company has been moving ahead with installations of its Hitachi Proton Beam system since receiving FDA clearance in 2008.
The system is equipped with a compact synchrotron accelerator, which the company says allows for beam delivery at selected energy levels, with significantly less neutron generation, and is used by facilities including the MD Anderson Cancer Center and Mayo Clinic sites in Minnesota and Arizona.
The company is currently working on an installation at Sibley Memorial Hospital, a member of Johns Hopkins Medicine, in Washington, D.C., and hopes to start treating sometime in late 2019, said Sash Matsumoto, general manager at Hitachi America.
The company also announced late last year that it will install a system at Clinica Universidad de Navarra (CUN) in Madrid, Spain.
Matsumoto noted that the lead time for constructing proton therapy facilities is getting shorter and the overall cost is coming down, though facilities are mainly focusing on one- or two-room centers instead of multiple rooms because of the investment.
“Not every hospital can afford a multi-room proton system,” Matsumoto said. “I think every vendor is doing everything we can to lower the cost and, hopefully, it will allow more hospitals to have the technology. With more clinical trials providing evidence, it’s become more and more clear that for certain tumors proton is the best treatment and it’s something that every patient deserves to have access to.”
IBA
Since the company’s ProteusPLUS treated its first patient in 2001 and its smaller ProteusONE was first used in 2014, IBA has been rapidly deploying its technology. In the last six months, IBA completed four installations of the ProteusONE – which has pencil beam scanning and cone beam CT – around the world.
The company’s goal is to make the system such that it could be used for a wide variety of indications, with the ability to treat complex tumors, including moving ones, using respiratory gating systems and better imaging, said Nicolas Denef, IBA’s product management director.
Denef said the ProteusONE, which has a smaller footprint and offers an affordable solution for a one-room proton therapy system, has become the preferred technology as fewer facilities have built multi-room facilities.
Several years ago, developers thought that having four to five rooms would be more financially beneficial, but patient recruiting was more difficult for more remote Proton Therapy Centers.
“There was an idea that if you build it they would come,” Denef said. “Which happened for some, but not for the ones that were far away from city centers.”
The company’s IBA Dosimetry solution decreases the time needed for beam data commissioning and accelerator QA, Denef said.
In the future, IBA is looking to continue to improve imaging and introduce improved workflows, Denef said. The ultimate goal is to do adaptive treatment, using the cone beam CT to replan quickly just before treatment starts.
Mevion Medical Systems
Earlier this year, Mevion received FDA clearance for HYPERSCAN pencil beam scanning for its compact MEVION S250i Proton Therapy System. Shortly afterward, MedStar Georgetown University Hospital in Washington, D.C., became the first to treat a patient with the system, which combines pencil beam scanning with a single-room machine.
The system features what Mevion calls the Adaptive Aperture proton MLC, which the company says generates layer-by-layer specific beam collimation and blocking with beam sharpening performance identical to conventional proton therapy apertures.
“The combination of HYPERSCAN and Adaptive Aperture moves the science of proton therapy ahead to even sharper dose distributions,” said Skip Rosenthal, vice president of clinical systems for Mevion Medical Systems.
The company has two more systems in deployment, one in Europe and another system being installed in the U.S. in the fall.
An important aspect of the HYPERSCAN is the ability to have support from advanced treatment planning, and the company has a collaboration with RaySearch. Currently, centers will be using RayStation, the RaySearch treatment planning system to provide clinicians with Monte Carlo-based planning for HYPERSCAN. Rosenthal said that by the end of the year, RaySearch will release the ability to plan and treat using the full multilayer collimation capabilities of Adaptive Aperture.
Rosenthal said Mevion also is integrating cone beam CT and CT on rails with the system sometime this year.
“The ability to use that in a proton room has been slow to materialize,” Rosenthal said.
P-Cure
P-Cure has marketed its gantry-less solution, and the company is planning to soon publicize results from use of the P-Cure system on patients being treated for lung, brain, eye and other types of cancer at the Northwestern Medicine Chicago Proton Center.
“The objective is to show … that patients can be treated even better without a gantry, in the seated position,” said Michael Marash, P-Cure’s chief executive officer. “When a patient is seated, the magnitude of the inner organ motion is minimized, enabling it to direct the beam to the tumor with much greater precision. There are also fewer equipment and construction costs, as a gantry needs three floors.”
At the Chicago center, P-Cure enabled treating in their open registry study. Next year, the company will look to show the effects of the system on treating several other tumor sites including, head and neck, breast, and liver cancers.
The company is also starting to explore international markets and has received a positive response from facilities in India and China, Marash said. The company is building a new facility in the center of Israel to ship both west and east.
ProTom International Holding Corporation
ProTom has made moves into the Asia Pacific market and was recently awarded the contract for the Australian Bragg Centre for Proton Therapy, the first proton therapy center in Australia, to be housed in a massive new health complex expected to break ground in the second quarter of next year in Adelaide.
“The U.S. market is more mature,” said Stephen Spotts, chief executive officer of ProTom International. “As cancer providers are looking at adding proton therapy, more providers are looking at single room systems.”
ProTom's Radiance 330 Proton Therapy System – which received FDA clearance in March 2014 – uses one synchrotron accelerator for multiple rooms, which is different from the company’s competitors, except for Hitachi, Spotts said. The center in South Australia is using renewable energy, which is one of the reasons they chose ProTom, which uses a synchrotron, with varied acceleration levels, as opposed to a cyclotron, where the acceleration is constant.
“Our energy consumption is about 30 percent of a multiple-room cyclotron,” Spotts said.
Radiation oncology
Like proton therapy, radiation oncology companies have shown greater interest in simplifying their systems while also providing greater specificity. What follows is a look at some of the newest software and hardware solutions from companies in the conventional radiation oncology space.
Accuray
At this month’s ASTRO annual meeting, Accuray will be introducing the CyberKnife VOLO optimizer for the CyberKnife robotic radiation therapy system. The system simplifies the process of creating treatment plans using a new next-generation optimization algorithm, said Corey Lawson, vice president of product strategy at Accuray.
“It’s a state-of-the-art optimizer that resets the bar for user experience,” Lawson said.
Lawson said VOLO should reduce optimization time, or the time to do calculations, by 95 percent. So, a lung cancer case that may have taken close to an hour with the previous optimizer will now take a few minutes. It will also reduce delivery time by more than 20 percent.
Faster treatment planning means clinicians can try different ways of planning a case with much greater efficiency, Lawson said.
“With the ability to push a plan harder than ever before, will likely come the ability to get better plans than previously thought possible with the CyberKnife System,” Lawson said. “We expect our clinical partners to readily adopt this new technology because of the clinical workflow and plan quality benefits that will be quickly realized.”
Accuray also recently introduced CTrue IR imaging on its Radixact Treatment Delivery System. CTrue IR integrates a new iterative reconstruction algorithm which improves soft tissue contrast, while also reducing noise in scanned image sets.
"It does all of this with the same low-dose imaging and fast scan time we've always had,” Lawson said. “Furthermore, it is not affected by metal artifacts, nor in larger patients, the obscuring effects of electron starvation. We are truly improving user experience and clinical utility with this new release for our Radixact System.”
The company is also planning to show Synchrony motion compensation on the Radixact Treatment Delivery System, which is 510(k) pending at the time of this article.
Lawson stressed that the new feature is very different than conventional gating solutions. It will use an algorithm to adjust the beam to follow a moving target throughout its motion cycle, in real-time.
Brainlab
Brainlab released a newer version of its Elements Multiple Brain Mets SRS treatment planning software for focused stereotactic radiosurgery. The updated software provides new treatment strategies to treat brain metastases and adds new contouring and response assessment tools to increase utilization, said Bogdan Valcu, director of clinical research at Brainlab.
The previous version of Elements Multiple Brain Mets SRS was only planning for a single fraction radiosurgery, or the treating oncologist would have to specify one fractionation strategy, Valcu explained. With the new version of the software, you can combine multiple strategies, with one tumor receiving a single fraction and one receiving multiple fractions.
“We just want to provide more flexibility within this application, enabling everything from single fraction radiosurgery to fractionated SRS prescriptions customized to the individual patient disease and irrespective of tumor size,” Valcu said. “Using Elements Contrast Clearance Analysis, we can now detect earlier whether or not patients are failing therapy and require re-treatment. Timely re-treatment is critical for maximizing survival profiles for brain metastasis patients and so is the ability to distinguish between tumor and radiation effect.”
The spine is another key target for Brainlab, and in March 2017 the company released Elements Spine SRS. First patient treatments began in Argentina, Germany and Italy followed by the first patient treatment in the U.S. in August of 2018.
Elements Spine SRS works with CT and MR scans to calculate the clinical target volume using international spine radiosurgery consortium consensus guidelines, and takes less time using Monte Carlo simulations.
“The software offers automatic treatment planning, which helps raise overall plan quality with consistent adherence to desired treatment protocol. In a half hour clinicians can go from zero to treatment plan,” Valcu said. “Planning steps that would have taken clinicians two to three hours to perform in the past are now performed in under 10 minutes and physician involvement time is reduced to just a few minutes.”
Brainlab specializes in customized algorithms for the treatment site, working around the challenges of the spinal column.
In March 2017 Brainlab also released Elements Cranial SRS for treating primary tumors in the brain. The software corrects the cranial distortion from MR scans, Valcu said.
“Planning automation is paramount for this software, starting with tools to correct MRI distortions and improve co-registration, to planning CT and continuing with a comprehensive risk organ definition based on a tissue-class universal atlas,” Valcu said.
The company plans to continue developing indication-specific solutions and plans to focus on metastases in the lung and liver and other body sites.
Elekta
Elekta has mainly been promoting its Elekta Unity MR-linac, which received a CE mark in June 2018 (the company submitted for FDA 510(k) clearance in beginning of August).
In August, the first treatment of a cancer patient with a recurrence in a pelvic lymph node with the Unity was completed in the Netherlands. The concentrated high-dose treatment took 10 days, significantly less than typical radiation therapy treatments that require up to 30 fractions, said Ioannis Panagiotelis, the chief Marketing and sales officer for Elekta.
On the software side, Elekta released a product called AQUA, which standardizes and automates clinical quality assurance tests.
The company also entered into two agreements. One is with IBM, to allow its Watson for Oncology to pair with Elekta’s MOSAIQ, oncology information system and personalize treatment with artificial intelligence (AI). Elekta also partnered with Palabra to allow clinicians to use voice commands for MOSAIQ to expedite clinical operations and reduce the time needed for data entry.
“Our customers have access to the latest and greatest when it comes to software solutions that have been developed by other parties,” Panagiotelis said.
RaySearch Laboratories
Late last year, RaySearch Laboratories released RayCare, its new oncology information system (OIS).
Johan Löf, president and chief executive officer of RaySearch Laboratories, said RayCare is a “completely new take on” the OIS, with an “advanced architecture that is extremely resilient and scalable.”
Users have full access to the system through Android or iOS devices and the company introduced a structured way of handling workflows. If a user is logged on in a certain role, they will only see the tasks relevant to that role. The company has been updating RayCare to add functionality, including billing.
In June, the company released a new version of its RayStation treatment planning system that includes full planning capabilities for the TomoTherapy and Radixact systems from Accuray, and support for Accuray’s TomoDirect and TomoHelical delivery modes.
Later this year, RaySearch Laboratories plans to release an auto-contouring product that applies deep learning, with a database of “well-contoured” patients that will allow the process to be completely automated.
Varian
Varian recently released version 2.0 of its Halcyon radiotherapy system, which debuted at last year’s ESTRO meeting in Vienna.
The new version adds additional imaging modalities – kV cone beam CT and iterative cone beam CT, which Tracey Fisher, director of marketing for the Americas oncology division at Varian, said brings a “diagnostic-like” quality to the images.
“What is unique about Halcyon is the speed,” Fisher said. “You can get kV volumetric images in as few as 17 seconds. It’s better soft-tissue imaging and target delineation. The more you can see, the better is the potential treatment.”
The Halcyon is field upgradable, and since the launch Varian has taken 98 orders for the system worldwide.
The new version of Varian’s Eclipse treatment planning system, Eclipse 15.5, enables many of the features of the Halcyon 2.0 and a feature that allows clinicians to more easily adapt a treatment plan from one machine to another. Additionally, Eclipse 15.5 features Multicriteria Optimization (MCO), which allows clinicians to explore what happens when different clinical criteria are varied, such as the degree to which particular organs are spared versus coverage of the targeted tumor.
In April, Varian announced a new version of its Velocity cancer imaging software. Called Velocity 4.0, it has several new capabilities, including RapidSphere image-guided Y90 dosimetry, which offers an image-guided dosimetry solution for Y90 Selective Internal Radiation Therapy (SIRT) and is a method for tracking absorbed dose, confirming that the treatment was delivered as planned.
The software also offers new features including Velocity M3i, which allows clinicians to use images from multiple modalities – CT, MR, PET, SPECT and ultrasound – for contouring, and Velocity ARIA Sync, which automatically synchs with Varian’s ARIA oncology information system and Eclipse treatment planning system.
A feature called Velocity Tumor & Dose Tracking, which can track radiation dose of multiple treatments and different types of therapies, allows clinicians to verify volumetric tumor changes and a patient’s cumulative dose history.
“This allows them to more easily account for prior treatments and their impact on current or future treatments,” Fisher said.
ViewRay
Last year, ViewRay’s big release was the MRIdian Linac, which combines a linear accelerator with an MR scanner and was cleared by the FDA in February 2017. The company later announced imaging improvements under development that double the signal-to-noise ratio, frame rate and resolution without raising the field strength, said Jim Dempsey, ViewRay's chief scientific officer.
The device uses what Dempsey called a “proprietary approach” to compressed sensing.
“We think there are some very impressive imaging improvements, under development,” Dempsey said. “Speed, resolution and SNR are potentially improved by a factor of three.”
Elekta, which released a similar MR-linac, called the Elekta Unity, uses an MR scanner with a higher field strength
The second generation is available via an upgrade, and Dempsey said some customers with service agreements have already purchased upgrades.
“Their argument has been you need high field strength,” Dempsey said. “Elekta and Philips made a decision to sacrifice dose quality for a better image. As a small team, we work on impossible problems, such as how to make a low-field MRI work like a high-field MRI.”
Dempsey notes that the clinical data shows that the MRIdian can treat cancer with lower toxicity.
“The biggest headlines for us are the clinical data,” Dempsey said.
Xstrahl
In December 2017, Xstrahl launched the new RADiant X-ray radiation therapy system with an FDA 510(k) clearance, which was followed by a CE mark in March 2018.
The RADiant system offers two types of treatment – electronic brachytherapy (eBX) and superficial radiotherapy (SRT), which is different from conventional radiotherapy systems currently on the market, according to Adrian Treverton, chief operating officer of Xstrahl Inc. This allows customers to increase their throughput and allows for adoption by the American dermatological market, which cannot use the high-powered radiotherapy systems currently on the market.
“The radiotherapy industry current has two main trends – a significant increase in skin cancers and skin conditions worldwide, and increased pressures on departments and clinics to treat patients in cost- and time-effective ways,” said Treverton. “The RADiant offers a cost-effective treatment platform treating not only skin cancers, but also non-malignant skin conditions, keloids and dermatological conditions, allowing for reduced pressure on oncology departments, and an increase of client base for dermatological clinics.”
RADiant is currently installed at four sites across the continental U.S., both in radiotherapy oncology departments and dermatology clinics. The RADiant will also soon be released in Europe, South America and China, before a full worldwide rollout.