August New Product Showcase
August 05, 2016
Mobile-Connected Ultrasound Urologic Visualization Device
Signostics has received FDA 510(k) clearance for Uscan, the first smart mobile-connected ultrasound visualization device targeted at urologic care. Using algorithms from the science of computer vision, Uscan actively recognizes the 3-D contours of the bladder. It acquires up to 256 bladder slices, 32 times more than conventional bladder scanners, resulting in industry-leading accuracy. It also provides real-time ultrasound imaging of the kidneys, pelvic floor, prostate, gallbladder, bladder stones and catheter emplacement, for quick and easy visual tracking and observation. It is compatible with Android operating systems and has built-in WiFi and Bluetooth connectivity that enables fast and reliable image management and interoperability with electronic health record (EHR) systems.
Wireless Remote Patient Monitor
CareTaker Medical, a pioneer in wireless remote patient monitoring devices, received 510(k) clearance for the company’s Wireless Continuous Non-Invasive “Beat-by-Beat” Blood Pressure and Heart Rate Monitor based on patented finger cuff technology. The wearable CareTaker monitor enables uninterrupted wirefree and electrode-free vital signs monitoring. Using a low-pressure finger cuff, CareTaker’s patented Pulse Decomposition Analysis technology non-invasively measures continuous beat-by-beat blood pressure with accuracy exceeding AAMI requirements, and measures heart rate as accurately as a 3-lead ECG for remote display on the CareTaker secure web portal or other wireless devices. CareTaker’s onboard cellular and Bluetooth communication capabilities allow for simple setup and deployment as well as seamless integration with other devices.
Vitamin and Medicine Management System
HERO, a Brooklyn-based health technology company, debuts the HERO smart appliance, the world’s first household companion for managing vitamins and medicines. HERO stores up to 10 different pills, dispenses them on schedule or on demand, and monitors consumption. The HERO mobile app allows users to receive reminder notifications when it’s time to take a pill, and offers caregivers peace of mind by alerting them when a dose is missed. The HERO app provides real-time medication information and actionable data to help users stay on track. The app is now available on iOS and Android.
MR-Conditional Pacing System
Boston Scientific has received FDA approval for a suite of products deemed safe for use in an MRI environment. The ImageReady MR-Conditional Pacing System, which includes ACCOLADE MRI and ESSENTIO MRI pacemakers, as well as the new INGEVITY MRI pacing leads, is designed to treat bradycardia. Patients implanted with the full system are able to receive full-body MR scans in 1.5 Tesla environments when conditions of use are met. The newly approved family of INGEVITY MRI pacing leads includes active and passive fixation models. This marks the first time a passive fixation pacing lead is approved for U.S. patients undergoing MR scans.
Single-Chamber ICDs for Detecting Atrial Fibrillation
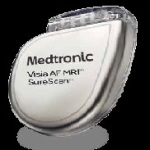
Medtronic announced it has received FDA approval for the Visia AF MRI SureScan and Visia AF single-chamber implantable cardioverter defibrillators. The Visia AF devices can detect previously undiagnosed and/or asymptomatic atrial fibrillation and monitor recurrent AF, while treating life-threatening rhythms in the lower chambers of the heart.. The Visia AF ICDs include a proprietary algorithm that detects AF episodes (without a lead in the atrium) and captures AF frequency and duration, information that helps physicians identify AF and tailor treatment for these patients. More than half of all new ICD implants in the U.S. are single-chamber devices.
Radixact System
Accuray Incorporated has received 510(k) clearance for its Radixact™ Treatment Delivery Platform and its new treatment planning and data management systems, Accuray Precision Treatment Planning System and iDMS Data Management System. These next generation hardware and software solutions, together, make up the new Radixact system. The system features a more powerful linear accelerator, MVCT imaging and helical treatment delivery, so clinicians can apply highly conformal and homogenous dose distributions to any target volume, while precisely sparing normal healthy tissue during each treatment fraction. The new Accuray Precision Treatment Planning System with smart, automated workflows and midcourse decision-making tools enables clinicians to adapt delivery to changes in tumor size, shape and location within the patient.
Signostics has received FDA 510(k) clearance for Uscan, the first smart mobile-connected ultrasound visualization device targeted at urologic care. Using algorithms from the science of computer vision, Uscan actively recognizes the 3-D contours of the bladder. It acquires up to 256 bladder slices, 32 times more than conventional bladder scanners, resulting in industry-leading accuracy. It also provides real-time ultrasound imaging of the kidneys, pelvic floor, prostate, gallbladder, bladder stones and catheter emplacement, for quick and easy visual tracking and observation. It is compatible with Android operating systems and has built-in WiFi and Bluetooth connectivity that enables fast and reliable image management and interoperability with electronic health record (EHR) systems.
Wireless Remote Patient Monitor
CareTaker Medical, a pioneer in wireless remote patient monitoring devices, received 510(k) clearance for the company’s Wireless Continuous Non-Invasive “Beat-by-Beat” Blood Pressure and Heart Rate Monitor based on patented finger cuff technology. The wearable CareTaker monitor enables uninterrupted wirefree and electrode-free vital signs monitoring. Using a low-pressure finger cuff, CareTaker’s patented Pulse Decomposition Analysis technology non-invasively measures continuous beat-by-beat blood pressure with accuracy exceeding AAMI requirements, and measures heart rate as accurately as a 3-lead ECG for remote display on the CareTaker secure web portal or other wireless devices. CareTaker’s onboard cellular and Bluetooth communication capabilities allow for simple setup and deployment as well as seamless integration with other devices.
Vitamin and Medicine Management System
HERO, a Brooklyn-based health technology company, debuts the HERO smart appliance, the world’s first household companion for managing vitamins and medicines. HERO stores up to 10 different pills, dispenses them on schedule or on demand, and monitors consumption. The HERO mobile app allows users to receive reminder notifications when it’s time to take a pill, and offers caregivers peace of mind by alerting them when a dose is missed. The HERO app provides real-time medication information and actionable data to help users stay on track. The app is now available on iOS and Android.
MR-Conditional Pacing System
Boston Scientific has received FDA approval for a suite of products deemed safe for use in an MRI environment. The ImageReady MR-Conditional Pacing System, which includes ACCOLADE MRI and ESSENTIO MRI pacemakers, as well as the new INGEVITY MRI pacing leads, is designed to treat bradycardia. Patients implanted with the full system are able to receive full-body MR scans in 1.5 Tesla environments when conditions of use are met. The newly approved family of INGEVITY MRI pacing leads includes active and passive fixation models. This marks the first time a passive fixation pacing lead is approved for U.S. patients undergoing MR scans.
Single-Chamber ICDs for Detecting Atrial Fibrillation
Medtronic announced it has received FDA approval for the Visia AF MRI SureScan and Visia AF single-chamber implantable cardioverter defibrillators. The Visia AF devices can detect previously undiagnosed and/or asymptomatic atrial fibrillation and monitor recurrent AF, while treating life-threatening rhythms in the lower chambers of the heart.. The Visia AF ICDs include a proprietary algorithm that detects AF episodes (without a lead in the atrium) and captures AF frequency and duration, information that helps physicians identify AF and tailor treatment for these patients. More than half of all new ICD implants in the U.S. are single-chamber devices.
Radixact System
Accuray Incorporated has received 510(k) clearance for its Radixact™ Treatment Delivery Platform and its new treatment planning and data management systems, Accuray Precision Treatment Planning System and iDMS Data Management System. These next generation hardware and software solutions, together, make up the new Radixact system. The system features a more powerful linear accelerator, MVCT imaging and helical treatment delivery, so clinicians can apply highly conformal and homogenous dose distributions to any target volume, while precisely sparing normal healthy tissue during each treatment fraction. The new Accuray Precision Treatment Planning System with smart, automated workflows and midcourse decision-making tools enables clinicians to adapt delivery to changes in tumor size, shape and location within the patient.