March's new product showcase
March 22, 2013
by Sean Ruck, Contributing Editor
To view the full product description, click the link following the story.
CleanShield Endoscope Storage Cabinet
CS Medical LLC has released the CleanShield Endoscope Storage Cabinet. CleanShield is constructed completely of thermally fused polypropylene that can be disinfected easily and is non-hygroscopic. The cabinet contains special hanging mounts that allow each endoscope to hang vertically while being bathed in clean, filtered air. Designed to accommodate up to nine disinfected endoscope probes, it’s secured with a locking epoxy-coated steel door.
Click here for the full product description.
The Pathogon UV Disinfection System
New from STERIS, the system features germicidal ultraviolet light that may be used as part of a cleaning and disinfection program to reduce or kill pathogens on environmental room surfaces. Used after terminal cleaning, it helps minimize human error on missed surfaces and insufficient chemical contact times, and requires no significant special training for operation.
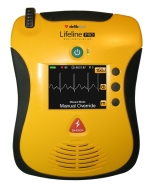
Lifeline PRO and Lifeline ECG
Defibtech’s Lifeline PRO and Lifeline ECG automated external defibrillators (AEDs) have been cleared for sale in the U.S. market by the Food and Drug Administration. Operating in semi-automatic or “AED” mode, both the Lifeline PRO and ECG AEDs can display either an ECG or video instructions for the rescuer. In this mode, the AED determines whether or not a shock is warranted through field-proven arrhythmia detection technology. Both models’ video and audio provide guidance on where to place the defibrillating pads and give step-by-step lifesaving instructions. For example, when the audio says, “Place pads on patient’s chest,” the video shows exactly where to place the pads.
Click here for the full product description.
ENSEAL G2 Articulating Tissue Sealer
To address surgeons’ need for strong sealing in tight spaces where access is limited, Ethicon Endo-Surgery, Inc. recently announced the 510(k) clearance from the U.S. Food and Drug Administration for the ENSEAL G2 Articulating Tissue Sealer. ENSEAL G2 Articulating is the first articulating advanced energy device designed to allow surgeons to take a perpendicular approach to seal vessels up to 7mm in diameter and lymphatics through a 5mm port.
Click here for the full product description.
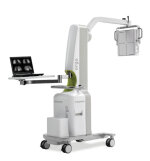
ergo Imaging System nuclear medicine camera
Digirad Corporation recently announced that it received U.S. FDA 510(k) market clearance to expand the clinical applications for their system. The additional clearance includes indications for lymphatic scintigraphy and parathyroid scintigraphy, both nuclear diagnostic imaging tests that aid in the evaluation of lesions in the breast and other small body parts. The system features a large field of view solid-state detector. Five collimator options provide the capability to provide outstanding imaging quality for a wide range of procedures with energy ranges from 50 to 350 keV. The open gantry, long 48” reach and multiple compound motions provide enhanced positional flexibility to address various applications and patient settings. Lightweight and portable, it can be easily moved throughout the hospital maximizing the system’s utility and convenience.
Click here for the full product description.
EDGE radiosurgery system
The U.S. Food and Drug Administration has granted Varian Medical Systems clearance for a new radiosurgery system that the company said will improve tumor tracking and motion management when delivering high doses of radiation to patients during treatment. The new EDGE radiosurgery suite includes five technologies: a flexible treatment couch to position patients optimally; a real-time imaging package that also includes expanded use of fluoroscopy and 4D cone-beam CT; an integrated intracranial radiosurgery package; and several enhancements to Varian’s real-time tracking system Calypso. The Calypso anchored Beacon transponder for implantation — or position signaling device to track motion during treatment in the lung — is still pending 510(k).
Click here for the full product description.
CleanShield Endoscope Storage Cabinet
CS Medical LLC has released the CleanShield Endoscope Storage Cabinet. CleanShield is constructed completely of thermally fused polypropylene that can be disinfected easily and is non-hygroscopic. The cabinet contains special hanging mounts that allow each endoscope to hang vertically while being bathed in clean, filtered air. Designed to accommodate up to nine disinfected endoscope probes, it’s secured with a locking epoxy-coated steel door.
Click here for the full product description.
The Pathogon UV Disinfection System
New from STERIS, the system features germicidal ultraviolet light that may be used as part of a cleaning and disinfection program to reduce or kill pathogens on environmental room surfaces. Used after terminal cleaning, it helps minimize human error on missed surfaces and insufficient chemical contact times, and requires no significant special training for operation.
Lifeline PRO and Lifeline ECG
Defibtech’s Lifeline PRO and Lifeline ECG automated external defibrillators (AEDs) have been cleared for sale in the U.S. market by the Food and Drug Administration. Operating in semi-automatic or “AED” mode, both the Lifeline PRO and ECG AEDs can display either an ECG or video instructions for the rescuer. In this mode, the AED determines whether or not a shock is warranted through field-proven arrhythmia detection technology. Both models’ video and audio provide guidance on where to place the defibrillating pads and give step-by-step lifesaving instructions. For example, when the audio says, “Place pads on patient’s chest,” the video shows exactly where to place the pads.
Click here for the full product description.
ENSEAL G2 Articulating Tissue Sealer
To address surgeons’ need for strong sealing in tight spaces where access is limited, Ethicon Endo-Surgery, Inc. recently announced the 510(k) clearance from the U.S. Food and Drug Administration for the ENSEAL G2 Articulating Tissue Sealer. ENSEAL G2 Articulating is the first articulating advanced energy device designed to allow surgeons to take a perpendicular approach to seal vessels up to 7mm in diameter and lymphatics through a 5mm port.
Click here for the full product description.
ergo Imaging System nuclear medicine camera
Digirad Corporation recently announced that it received U.S. FDA 510(k) market clearance to expand the clinical applications for their system. The additional clearance includes indications for lymphatic scintigraphy and parathyroid scintigraphy, both nuclear diagnostic imaging tests that aid in the evaluation of lesions in the breast and other small body parts. The system features a large field of view solid-state detector. Five collimator options provide the capability to provide outstanding imaging quality for a wide range of procedures with energy ranges from 50 to 350 keV. The open gantry, long 48” reach and multiple compound motions provide enhanced positional flexibility to address various applications and patient settings. Lightweight and portable, it can be easily moved throughout the hospital maximizing the system’s utility and convenience.
Click here for the full product description.
EDGE radiosurgery system
The U.S. Food and Drug Administration has granted Varian Medical Systems clearance for a new radiosurgery system that the company said will improve tumor tracking and motion management when delivering high doses of radiation to patients during treatment. The new EDGE radiosurgery suite includes five technologies: a flexible treatment couch to position patients optimally; a real-time imaging package that also includes expanded use of fluoroscopy and 4D cone-beam CT; an integrated intracranial radiosurgery package; and several enhancements to Varian’s real-time tracking system Calypso. The Calypso anchored Beacon transponder for implantation — or position signaling device to track motion during treatment in the lung — is still pending 510(k).
Click here for the full product description.